Saison 2
20 épisodes
(1 h 40 min)
1
2
3
4
5
6
7
8
9
10
Épisodes
Modifier ma progression
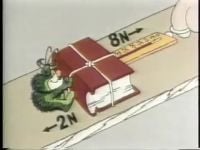
S2 E10 • Mesure de la température
Given three bathtubs of varying temperature, the star of the show ""blunts"" his feet so that they can't tell temperature. Sure they can't. The human body can only tell changes in temperature in comparison to what it had been used to. It's up to an independent device: a thermometer and the scale devised by Anders Celsius.